Hyularonic Acid in the Treatment of Osteoarthritis
A review of how hyularonic acid is used in the treatment of osteoarthritis, and the efficacy thereof
Doctors have been using hyaluronic acid (HA) as therapeutic treatment of osteoarthritis for decadess. There are many different hyaluronic acid treatment options, and a wide range of therapeutic products are available for clinical application. Numerous clinical trials and scientific papers have analyzed the clinical efficacy of hyaluronic acid in the treatment of osteoarthritis.
Bowman et al. (1) presented the most recent systematic review of all the available, scientifically validated publications of randomized clinical trials using different hyularonic acid treatments. This publication provides a comprehensive overview of the current status of different treatment options and clinical recommendations.
Osteoarthritis: the facts
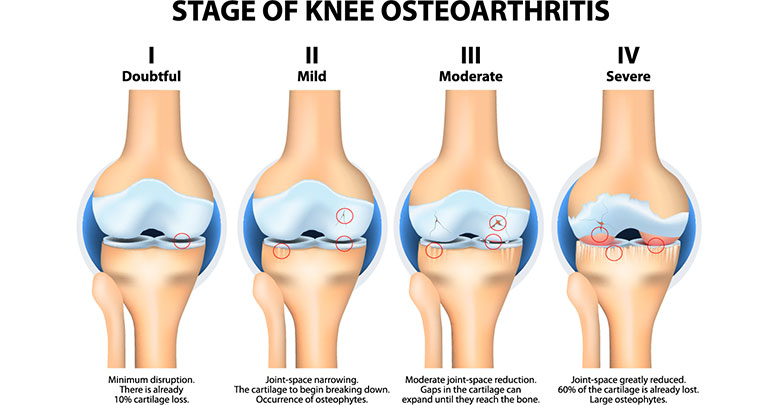
Osteoarthritis is a degenerative joint disease which most often affects the large load bearing joints and also the hands. In the USA alone, more than 50 million patients suffer from osteoarthritis (2). The main symptoms of osteoarthritis are joint pain, stiffness, and limited mobility. These symptoms often lead to reduced physical activity, resulting in further physical and mental co-morbidities such as obesity and depression (3). The exact pathology of osteoarthritis is not yet fully understood, due to the complexity of the disease and the diversity of risk factors – for example age, gender, hormones, weight, and genetic predisposition – which either alone or in combination may play an important role (4). Most of the therapeutic treatments for osteoarthritis target the most important symptom of pain, and provide varying levels of pain relief. The spectrum of therapies range from non-pharmaceutical treatments such as patient education, physical therapy, rehabilitation, and psychological support; to pharmaceutical and surgical treatment options. Pain control medications, such as NSAID and COX-2 inhibitors are frequently used in the treatment of osteoarthritis. For the most severe cases opioids are often prescribed (5). Further non-surgical treatment options are the supplementation of chondroitin sulfate and glucosamine as well as the intra-articular administration (injections into the joint) of blood derived products like PRP (platelet rich plasma), corticosteroids and visco-supplements such as collagens and hyularonic acid. Despite some controversies the majority of the available clinical studies have proven that intra-articular injection of hyularonic acid is an efficient and safe method among all intra-articular treatments (6).
What is hyularonic acid (HA)?
Hyaluronate is a molecule, which naturally and abundantly occurs in the extracellular matrix and in synovial fluid. In other words it is a common molecule which is found in joint cartilage and the fluid which lubricates the joints. In synovial fluid the higher molecular weight HA molecules, with molecular mass ranging between 6500 – 10900 kD, are mostly present (7).

They main physiological function of hyularonic acid within synovial fluid is to provide viscosity and to serve as a lubricant between the joint surfaces. HA also binds free radicals and proteins, thereby regulating cellular activities and contributing to a healthy homeostasis (cellular balance) of the joints (8).
During the progression of osteoarthritis, the high weight HA molecules are broken down to smaller weight molecules – below 4500 kD – by hyaluronidase enzymes. Due to this breakdown process, HA molecules lose their lubricating characteristics and cannot provide the physiological vicso-elasticity to the joints. Some studies suggest that the individual progression of osteoarthritis may be connected to different activity levels of hyaluronidase enzymes (9).
Effects of hyularonic acid in the joint
The aim of intra-articular injection of high molecular weight HA, is to replace, or to mitigate the reduced function of naturally occurring and deteriorating HA. Injected hyularonic acid does not replace the full properties and physiological activities of the naturally occurring HA of the synovial fluid, but it seems to achieve sufficient pain relief via different mechanisms. These mechanisms include anti-inflammatory effects, maintaining the visco-elasticity and synthetizing proteoglycans and glycosaminoglycans (9).
Forms of administration
Osteoarthritis patients can use HA in two main ways: either taken orally, or as local injections. In addition, hyularonic acid is often used as a cell and drug carrier.
Orally taken hyularonic acid
In the case of oral administration, the hyularonic acid molecules are ingested, and absorbed as smaller polysaccharides. These polysaccharides are able to bind to specific receptors and to signal anti-inflammatory cytokine reactions. Oe et al. completed a systematic review on the effect of orally administered HA and found that patients had improved pain scores in comparison to placebo and the treatment has been proven as safe with no side effects in a 12 month follow up period. (10, 11, 12).
Intra-articular administration of hyularonic acid
In the case of intra- articular administration, high weight HA molecules are injected directly into the synovial fluid, in order to replace and restore the lost function of natural HA.
The injected HA provides many mechanisms leading to pain relief. These include improved lubrication, enhanced production of extracellular matrix proteins, altering cytokine reactions into ant-inflammatory response and maintaining cartilage thickness and surface smoothness (13).
Hyularonic acid as cell and drug carrier
When cross linked, HA hydrogels are excellent carrier matrices for different cell-based therapies. These crosslinked hydrogels are used in the fields of tissue engineering and regenerative medicine. Bone marrow, umbilical blood or fat derived stem cells (14), laboratory cultured autologous chondrocytes or different pharmaceuticals (15) such as growth hormones can be bound to HA in order to implant them into intra-articular cartilage. Either the implanted cells directly, or the locally migrated stem cells stimulated by the introduction of growth factors, provide tissue replacement and thereby reconstruct the deteriorated cartilage tissue.
Sources of hyularonic acid
Hyularonic acid products can be derived from animal tissues such as rooster comb, or are bio-fermented by genetically modified bacteria and produced in bioreactors. Most of the current clinically available HA products are produced by bacteria fermentation in bioreactors.
Clinical evidences for the use of HA
Knee Osteoarthritis
Over the last decades numerous clinical trials have been performed with different hyularonic acid products. The majority of studies investigated the clinical efficacy and safety of intra-articularly administrated HA in the knee joint. Most of the clinical trials and meta-analyses carried out, provided evidence for the superiority of intra-articularly administered HA with regards to pain relief and joint functionality when compared to placebo treatments (16, 17, 18). Xing D et al. confirmed these findings in the most recent systematic review from 2016. Based on this meta-analysis, the intraarticular administration of HA can be considered an effective and safe treatment option for knee osteoarthritis, which may delay the need for knee replacements (19).
Hip and ankle osteoarthritis
The treatment of osteoarthritis of the hip and ankle joints, with intra-articular HA administration has also been investigated in clinical trials. A meta-analysis of over twenty clinical trials for hip osteoarthritis concluded that intra-articular hyularonic treatment could be considered the best first line non-operative treatment option for hip osteoarthritis in terms of pain relief and joint functionality (20). Another systematic review on clinical trials with intra-articular HA administration provided similar evidence for ankle joint osteoarthritis (21).
Controversies over intra-articular HA treatments
Some earlier studies found no significant improvements in osteoarthritis symptoms after hyularonic acid treatment in comparison to placebo, as measured by internationally recognized clinical scores in the treatment of knee osteoarthritis. These studies however, used very high molecule weight (6000 kD and 10000 kD) HA products (22, 23).
The influence of molecule weight
These and other observations ignited scientific discussion and further evaluation of the question about how the different molecular weights of the administrated HA may influence the clinical outcomes and efficacy of treatment. Currently, the influence of the different molecular weights of intra-articularly injected HA remains debatable. Some studies, like Karatosun et al. 2005 found no significant difference between the clinical outcome after the treatment with high or low molecular weight HA (24). Berenbaum et al. on the other hand, demonstrated superior clinical data by using intermediate molecular weight HA versus low molecular weight HA (25).
Conclusion
Hyularonic acid treatment has shown clearly beneficial results, and has been proven as an effective conservative treatment option for osteoarthritis in numerous studies. The effects of improvement of intra-articular lubrication, pain relief, chondro protection and the promotion of anti-inflammatory processes seem to be especially well substantiated. Currently however, HA treatment is neither recommended or discouraged in the guidelines of the Osteoarthritis Research Society International (2012) nor in the guideline of the American College of Rheumatology (2013) due to the controversies and inconsistencies of the clinical trials (26, 27). The main concerns are related to the diversity of individual outcomes, which could relate to age, gender, co-morbidities or to different hyaluronidase enzyme activities. The uncertainty with regards to the possible influence of the different molecular weights of HA on the treatment outcomes needs to be investigated further. In addition more clinical trials need to be performed, in order to investigate the efficacy of hyularonic acid treatments in comparison to other clinically available conservative treatment options.
Hyularonic acid remains a very promising therapy option for the treatment of osteoarthritis. No doubt it will be further clinically proven with the availability of more clinical data and evaluations in the future.
References
1. Bowman S et al. 2018: https://www.researchgate.net/publication/323226761_Recent_advances_in_hyaluronic_acid_based_therapy_for_osteoarthritis
2. CDC 2012 https://www.ncbi.nlm.nih.gov/pubmed/24196662
3. Moskowitz RW 2009 https://europepmc.org/article/med/19817508
4. Herrero-Beaumont G et al. 2017 https://www.sciencedirect.com/science/article/abs/pii/S0378512216303048
5. Brosseau L 2017 https://journals.sagepub.com/doi/abs/10.1177/0269215517691084
6. da Costa BR et al. 2016 https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(17)30701-8/fulltext
7. Laurent TC et al. 1996 https://www.ncbi.nlm.nih.gov/pubmed/8724014
8. Ayhan E et al. 2014 https://www.scirp.org/(S(vtj3fa45qm1ean45vvffcz55))/reference/referencespapers.aspx?referenceid=1649103
9. Moreland LW et al. 2003 https://link.springer.com/article/10.1186/ar623
10. Oe M et al. 2016 https://link.springer.com/article/10.1186/s12937-016-0128-2
11. Asari A et al. 2010 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2915711/
12. Tashiro T et al 2012 https://www.ncbi.nlm.nih.gov/pubmed/23226979
13. Cooper C et al. 2017 https://onlinelibrary.wiley.com/doi/full/10.1002/acr.23204
14. Park YB et al. 2017 https://stemcellsjournals.onlinelibrary.wiley.com/doi/abs/10.5966/sctm.2016-0157%4010.1002/%28ISSN%292157-6580.yia-best-papers-2018
15. Hangody L et al. 2017 https://journals.sagepub.com/doi/full/10.1177/1947603517703732
16. Rivera F et al. 2016 https://jorthoptraumatol.springeropen.com/articles/10.1007/s10195-015-0388-1
17. Xin Y et al. 2016 https://onlinelibrary.wiley.com/doi/full/10.1111/1756-185X.12782
18. Chang KW et al. 2013 https://www.archives-pmr.org/article/S0003-9993(12)01104-5/fulltext
19. Xing D et al. 2016 https://www.nature.com/articles/srep32790
20. Piccirilli E et al. 2016 https://www.ncbi.nlm.nih.gov/pubmed/28066733
21. Chang KV et al. 2013 https://www.sciencedirect.com/science/article/abs/pii/S0003999312011045
22. Altman RD et al. 2004 https://www.sciencedirect.com/science/article/pii/S1063458404000858
23. Lundsgaard C et al. 2008 https://www.ncbi.nlm.nih.gov/pubmed/18415773
24. Karatosun V et al. 2005 https://www.semanticscholar.org/paper/Comparison-of-two-hyaluronan-drugs-in-patients-with-Karatosun-Unver/c40b58819bbb96372944581f828117baa5cba089
25. Berenbaum F et al. 2013 https://www.ncbi.nlm.nih.gov/pubmed/23194896
26. Osteoarthritis Research Society International (ORSI) Guideline https://www.oarsi.org/education/oarsi-resources/non-surgical-treatment-knee-oa
27. American College of Rheumatology Guideline https://www.rheumatology.org/Practice-Quality/Clinical-Support/Clinical-Practice-Guidelines