Tissue Engineering – the new Treatment for Osteoarthritis
For those with degeneration of cartilage in the knee, which is really the early stage of osetoarthritis, this may be the answer.
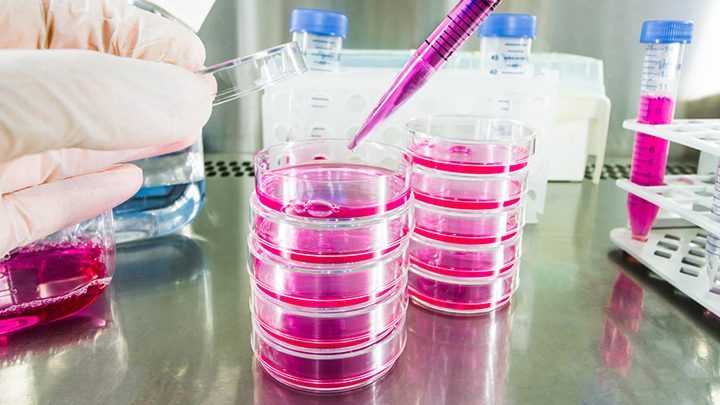
For those with degeneration of cartilage in the knee – which is really the early stage of osetoarthritis – tissue engineering may be the answer they are looking for. Tissue engineering is an interdisciplinary research and development field which uses the combinations of cell culture technology, biotechnology, biochemical engineering and biomaterials in order to improve or replace biological functions, tissues or organs.
What is tissue engineering?
The definition of tissue engineering covers a broad range of applications. In practice the term “tissue engineering” is mostly associated with the repair of, or replacement of tissues like skin, bone or cartilage. The term regenerative medicine is often used synonymously with tissue engineering. However, in general regenerative medicines emphasis is more on the use of stem cell technologies.

Is tissue engineering available for degeneration of cartilage in the knee?
Tissue engineering of joint cartilage has developed into a very successful and attractive research area, with numerous clinical applications already available for the treatment of degeneration of cartilage of the knee. In recent decades, scientists and surgeons have been developing different joint cartilage repair technologies for patients who suffer from degeneration of articular cartilage, such as osteoarthritis.
These modern, tissue engineered and cell-culture based implants are able to replace joint cartilage, and some of the latest technologies have started to show promising long-term results in repairing defective cartilage – caused by traumatic injuries or even by degenerative joint diseases like osteoarthritis. The tissue engineered implants provide pain relief and are helping patients to return to their pre-arthritis active lifestyle, to regain mobility, to go back to work and even to participate in sport again.

At the same time, these methods have the capacity to slow down the progression of the cartilage damage and to considerably delay or even avoid the necessity of a joint replacement.
ACI and MACI as treatments for cartilage defects
Currently, a variety of clinical methods – based on tissue engineering – are available for repairing joint cartilage defects, as in the case of osteoarthritis. These methods are often referred to as autologous chondrocyte implants (ACI) or matrix associated chondrocyte implants (MACI).
Both ACI and MACI cartilage repair procedures aim to restore the surface of an articular joint cartilage and to replace the defect with mechanically stable repair tissue, in order to prevent further degeneration and clinical progression of the condition.
In the case of ACI the cultured cells are provided in a solution while in the case of MACI the cells are already grown into a collagen or other matrix in the laboratories and are implanted as a cell seeded membrane.
ACI and MACI procedures take place in similar three stages:
Step 1 of tissue engineering
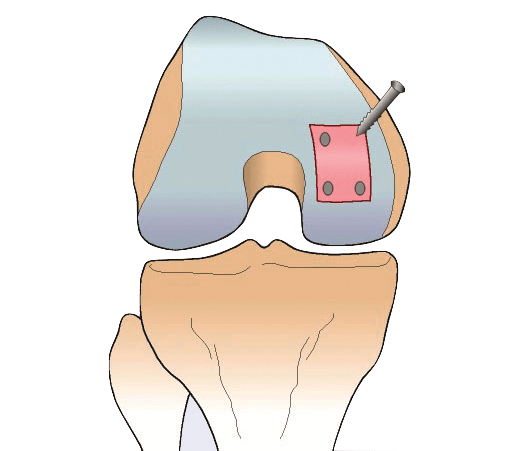
First, a very small cartilage specimen – smaller than a grain of rice – is arthroscopically collected from the patient’s healthy articular cartilage. It is collected from an area of the joint which is in a non-load-bearing area of the knee joint.
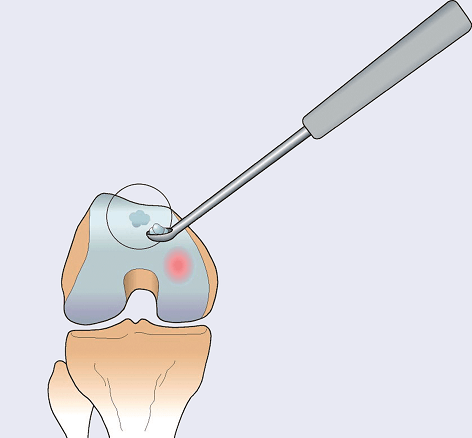
Step 2 of tissue engineering
Cartilage cells, also referred as chondrocytes, are extracted and transferred to an “in vitro” environment in specialized, super sterile laboratories where they grow and replicate. This process takes approximately four to six weeks, until the population of the cells has achieved a sufficient amount.

In the case of MACI the cells are transferred to a collagen membrane and are cultivated for a few more days on this collagen matrix.
Cartilage cell expansion in the laboratory is challenging due to the fact that the growing chondrocytes de-differentiate, which means that they lose their joint cartilage characteristics. Unfortunately, this also means that expanded cells tend to produce a scar like cartilage tissue which is rich in type I collagen instead of type II collagen which is the collagen necessary to maintain healthy cartilage tissue. The most recent cell culture technologies improve the re-differentiation of the laboratory cultured chondrocytes in order to stimulate them for the production of type II collagen already prior to transplantation.
Step 3 of tissue engineering
Finally, the patient undergoes a second surgery where the “in vitro” cartilage cells are applied to the damaged area.
In the case of ACI the chondrocytes are injected into the damaged area. A periosteum membrane, which is a thin layer of tissue surrounding the outer layer of the bones, is then applied to serve as protection for the newly engineered cells.
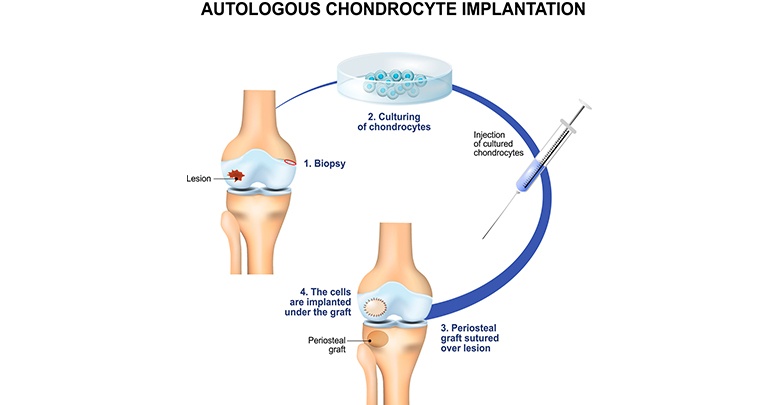
In case of MACI the combined cell-membrane implant is implanted and acts as a cell-matrix structure.
The transplanted cells grow in their new joint environment, and will develop further to form a new articular cartilage. This is the fundamental mode of action, and is how and why these cell-culture based implants help to stop, or slow down the progression of cartilage defects. This new articular cartilage leads to decreased pain, improved mobility, activity and quality of life of the patients. In the long term, the newly formed cartilage will be able to provide the necessary biomechanical and physiological protection for the joint, which can thereby prevent further surgery or even joint replacement.

Check out this TEDx talk by our scientific contributor, Dr Eszter Tánczos Olver, about the clinical achievements of tissue engineered cartilage implants.
References
1. Haleem AM et al. 2010 https://www.sciencedirect.com/science/article/abs/pii/S104866660900144X
2. Kessler MW et al. 2008 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2634176/
5. Trattnig S et al. 2005 https://www.sciencedirect.com/science/article/abs/pii/S0730725X05001979
6. Sridhar BV et al. 2015 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4758520/
7. Setayeshmehr M et al. 2019 https://www.liebertpub.com/doi/full/10.1089/ten.teb.2018.0245
8. Johnson K et al. 2012 https://science.sciencemag.org/content/336/6082/717
9. Brittberg M et al 2001 https://journals.lww.com/clinorthop/Abstract/2001/10001/Autologous_Chondrocytes_Used_for_Articular.31.aspx